Highlights
- The NIMML team combines animal and computational modeling in an innovative product development pipeline
- Successfully developed large animal models of infectious and immune-mediated diseases, including the development of pig models of IBD and H. pylori infection
- Staff composed of researchers, veterinarians, immunologists, toxicologists and pathologists
- Expertise in pre-clinical efficacy and safety testing of new drugs towards IND approval
- Mechanism of action discovered for naturally occurring compounds and new chemical entities by combining knockout mice and bioinformatics approaches.
- Integrated mouse colony management system and laboratory information management system for real-time data capture
Ongoing Efforts
The NIMML has over 15 years of experience in developing novel animal models of infectious, immune-mediated and metabolic chronic diseases. We have experience in mice, rats and pig models
TYPE II DIABETES ANIMAL MODELS
Diabetes mellitus type II is a chronic disease that is distinguished by insulin resistance and hyperglycemia (high concentrations of glucose in the blood) due to cells in the body not uptaking glucose.
Type II is a rapidly growing condition common in developed countries. Currently over 29 million Americans are diagnosed with Type II and out of all the cases of diabetes, Type II consists of 90% of the cases. Obesity, lifestyle, genetics, and previous medical conditions are the leading risk factors to Type II.
At NIMML we utilize animal models in order to simulate the proliferation of diabetic symptoms under conditions where potential biomolecular targets of medical interest can be manipulated and observed. In order to study these possible therapeutic targets and Type II NIMML has utilized the following mouse models.
- The db/db transgenic mouse model
- Cg-+Leprdb/+Leprdb/OlaHsd (db/db) strain mice are a model of obesity and diabetes caused by a deficiency in leptin receptor activity through gene knockdown. Due to this leptin deficiency, it causes the mice to become hyperphagic, obese and hyperglycemic. Obesity can be seen in mice after 5 weeks of age, followed quickly by hyperglycemia. The db/db mouse model insures quality genetically induced conditions in which diabetes symptoms can be accurately simulated.
- Controlled diet mouse model
- High-fat diet induced insulin resistance
- C57BL/6J strain mice are fed a high fat isocaloric and isonitrogenous AIN-93G diet where calories obtained from fat are increased substantially (16% more kcal from fat). Obesity from the diet drives insulin resistance over time. As this model is induced by an environmental manipulation rather than genetic, it may be considered more accurately representing the common human condition.
- Low-fat diet
- C57BL/6J strain mice were fed purified isocaloric and isonitrogenous diets that represented a low fat modification of the AIN-93G diet commonly used for the growth, pregnancy, and lactation phases of mice.
Selected Publications
Activation of PPAR gamma and alpha by punicic acid ameliorates glucose tolerance and suppresses obesity-related inflammation.
http://www.ncbi.nlm.nih.gov/pubmed/19828904
Abscisic acid synergizes with rosiglitazone to improve glucose tolerance and down-modulate macrophage accumulation in adipose tissue: possible action of the cAMP/PKA/PPAR γ axis
http://www.ncbi.nlm.nih.gov/pubmed/20207056
Catalpic acid decreases abdominal fat deposition, improves glucose homeostasis and upregulates PPAR alpha expression in adipose tissue.
http://www.ncbi.nlm.nih.gov/pubmed/18778878
Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets.
Data Figures
-

About
C. difficile experiments within NIMML follow a standard timeline of events. As mice are resistant to C. difficile infection with an intact intestinal flora, five days prior to infection with C. difficile (termed day -5), an antibiotic mixture (colistin, gentamycin, metronidazole, and vancomycin) is added to the water supply. Mice stay on the antibiotic water until day -3 of the timeline at which point mice will be placed back on standard water. On day -1, mice receive an intraperitoneal injection of clindamycin (32 mg/kg) to further remove any colonizing species. On day zero, mice are challenged with C. difficile through intragastric gavage. The strain VPI10463 is used for our studies. The model provides the opportunity to analyze an acute infection (through day 14) and a relapse period (through day 30).
The mouse model of C. difficile is able to be used to study a broad range of C. difficile related factors from the role of the microbiota to the balance of T cells to the activation of neutrophils to the formation of lesions. While the model requires the use of antibiotics to induce disease, the need for a reduced microbiota is not unlike the development of disease within humans, as most C. difficile infections are associated with antibiotic use. The model also provides the ability to study multiple events throughout the progression of disease from the initial colonization and response in early disease in days 1 through 3 and the peak of inflammation and disease on days 4 and 5 as well as the recovery phase and clearance of infection between days 8 and 10. Previously, we have utilized this model to establish systems level computational models of the host response to C. difficile, investigate the role of miRNA in the establishment of inflammation, and evaluate natural products for therapeutic efficacy.
Related Publications
Preclinical models for Infectious diseases: Respiratory pathogens
The NIMML has been working on Influenza models with several strains, being able to develop lung damage (i.e., epithelial necrosis, leucocyte infiltration), weight loss 10-20% and clinical disease. The virus strains we are using in mouse and pig challenge studies include:
- H1N1 strain A/Puerto Rico/8/34 (PR8)
- H3N2 strain A/Udorn/72
- H1N1 strain A/California/07/2009
NIMML can provide data on:
- Viral disease progression, weight loss
- Histology and immunohistochemistry
- Vaccine and therapeutic efficacy
- Immunological assays and flow cytometry
- Viral titers
- LD50 studies
Preclinical models for Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is characterized by two major clinical manifestations: Crohn’s disease (CD) and ulcerative colitis (UC). CD can affect any regions of the gastrointestinal (GI) tract from mouth to anus. In contrast, UC causes acute colonic inflammation. The NIMML offers expertise and services in mouse models of IBD such as trinitrobenzene sulfonic acid (TNBS), dextran sodium sulfate (DSS)-induced colitis, pan-enteritis due to the deficiency of interleukin-10 (IL-10 KO) and CD4+ T cell-induced colitis in adoptive transfer models. Whereas TNBS and DSS-induced colitis represent the more acute and faster induction of colonic tissue damage, the IL-10 KO and the CD4+ T cell induced colitis represent chronic models of IBD. We have also developed a model of inflammation-driven colorectal cancer (CRC) following treatment of mice with azoxymethane and DSS. The NIMML provides expertise and resources in these four preclinical models with more than 10 years of experience and results published in top gastroenterology journals. NIMML can provide data on
- Immunological changes at the gut mucosa and systemically
- Gut histological and immunohistochemical changes
- Transcriptomic analyses (RNAseq, ChipSep, miRNA)
- Pre-clinical efficacy, tolerability and weight loss
- Cellular and molecular profiling
Highlights
- Successfully developed a novel pig model of Helicobacter pylori infection
- Demonstrated for the first time strong cytotoxic CD8+ T cell responses during Helicobacter pylori infection in pigs, further confirming the role of H. pylori as an intracellular pathogen
- Identified the contribution of dysregulated immune responses to gastric inflammatory lesions following Helicobacter pylori infection, particularly by IFNγ-producing Th1 cells and inflammatory macrophages in particular
- Mined large RNAseq datasets from time course studies of macrophage-H. pylori co-cultures
- Identified novel immunological and metabolism genes modulated by H. pylori infection
Background and Epidemiology
Helicobacter pylori are gram-negative, miroaerophilic bacteria responsible for a variety of gastro-duodenal pathologies in the developed and developing world [1]. H. pylori is thought to be indigenous to the human population and is well adapted to colonize and persist in the human stomach. Infection is generally asymptomatic not causing clinical symptoms of those infected people. However, a small percentage of infected individuals will ultimately develop duodenal ulcers and gastric cancer due to the inability of the host immune system to clear the infection.
Pathogenesis
Dozens of bacterial factors are involved in H. pylori molecular pathogenesis (i.e. flagella, urease, catalase, neutrophil-activating protein Nap-A, vacA and cagA). These proteins have revealed many aspects of the relationships between the bacteria, the gastric mucosal surface, and the final outcome of the disease. Two of the most studied virulence factors are vacuolotoxin A (VacA) and the cytotoxin-associated gene A (cagA). Secreted VacA triggers pore formation in the cell membrane, endolysomal trafficking modification, cellular vacuolation, immune cell apoptosis and cell inhibition [2, 3]. CagA is an effector protein injected into the gastric epithelial cells by a type IV secretion system encoded by the cag pathogenicity island (cagPAI). Once inside the host cells, it localizes under the point of bacterial attachment and interacts with the protein zonulin (ZO-1) and the junctional adhesion molecule (JAM) [4]. CagA is then phosphorylated on EPIYA repeats in its phosphotyrosine (PY) region induce secretion of interleukin 8 (IL-8) [5].
Immune response towards H. pylori
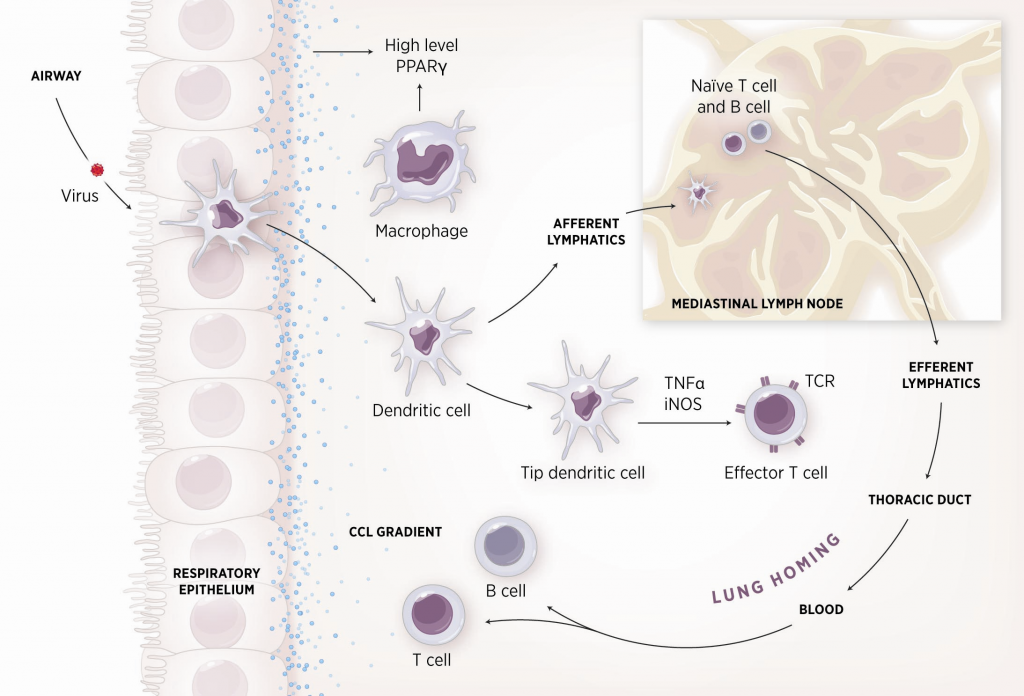
As shown in figure 1, the inflammatory response towards H. pylori is initiated through the interaction between the pathogen lipopolysaccharides (LPS) and the Toll-like receptors (TLR) expressed on gastric epithelial cells [6]. Once in the gastric lamina propria, H. pylori is mainly found inside macrophages where their interaction leads to macrophage activation and cytokine release [7]. Macrophages interact with T helper (Th) cells during infection and release cell-polarizing cytokines such as IL-17 [8]. In addition, H. pylori infection also involves neutrophils and increased antigen presenting activity of dendritic cells (DC) [9].
Modeling immunity to H. pylori
MIEP is basically focused on the characterization of the mechanisms underlying immune responses to enteric pathogens by integrating mathematical and computational modeling approaches with experimental data (Figure 2). The integration of modeling and experimental approaches provides unprecedented opportunities for systems-level knowledge discovery. Given the complexity of the host-H. pylori interactions at the systems level and the broad range of possible outcomes, our team has developed a computational model of the gastric mucosal immune response towards H. pylori infection. Currently, the model is able to predict the distinct time-dependent behavior of the three main CD4+ T cells (Th1, Th17 and iTreg) showing an increased Th17 response at the early stage of infection that switches to a Th1 predominance in the chronic phase of the infection.
References
1. Abdulrasheed A, Lawal OO, Abioye-Kuteyi EA, Lamikanra A: Antimicrobial susceptibility of Helicobacter pylori isolates of dyspeptic Nigerian patients. Tropical gastroenterology : official journal of the Digestive Diseases Foundation 2005, 26(2):85-88.
2. Amieva MR, El-Omar EM: Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology 2008, 134(1):306-323.
3. Tanaka S, Mizuno M, Maga T, Yoshinaga F, Tomoda J, Nasu J, Okada H, Yokota K, Oguma K, Shiratori Y et al: H. pylori decreases gastric mucin synthesis via inhibition of galactosyltransferase. Hepato-gastroenterology 2003, 50(53):1739-1742.
4.Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S: Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 2003, 300(5624):1430-1434.
5. Eaton KA, Kersulyte D, Mefford M, Danon SJ, Krakowka S, Berg DE: Role of Helicobacter pylori cag region genes in colonization and gastritis in two animal models. Infection and immunity 2001, 69(5):2902-2908.
6. Cullen TW, Giles DK, Wolf LN, Ecobichon C, Boneca IG, Trent MS: Helicobacter pylori versus the host: remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS pathogens 2011, 7(12):e1002454.
7. Ito T, Kobayashi D, Uchida K, Takemura T, Nagaoka S, Kobayashi I, Yokoyama T, Ishige I, Ishige Y, Ishida N et al: Helicobacter pylori invades the gastric mucosa and translocates to the gastric lymph nodes. Laboratory investigation; a journal of technical methods and pathology 2008, 88(6):664-681.
8. Dong C: TH17 cells in development: an updated view of their molecular identity and genetic programming. Nature reviews Immunology 2008, 8(5):337-348.
9. Zhuang Y, Shi Y, Liu XF, Zhang JY, Liu T, Fan X, Luo J, Wu C, Yu S, Chen L et al: Helicobacter pylori-infected macrophages induce Th17 cell differentiation. Immunobiology 2011, 216(1-2):200-207.
Highlights
- NIMML built a mathematical and computational model describing the immune response towards Helicobacter pylori infection.
- NIMML used two different strategies to model these reactions: ODE-based modeling and ABM-based modeling.
- Both strategies resulted in similar results, proving that the combination of ODE and ABM, when used as a complementary approach, can result in the identification of crucial nodes and in the generation of novel hypotheses.
Modeling of immune responses towards H. pylori - Ordinary differential equation model
Given the complexity of the host-H. pylori interaction and to facilitate a better understanding of the gastric mucosal immune response during H. pylori infection, we constructed a computational and mathematical model (Figure 1). The structural network of the model is comprised of three different compartments representing the effector sites: the gastric lumen, the epithelium and the gastric lamina propria plus a fourth inductor compartment representing the gastric lymph nodes (GLN). This network was used for equation-based and agent-based modeling efforts. The equation-based model is comprised by 24 species and 26 ordinary differential equations (ODE) that drive 43 reactions in both gastric mucosa and GLN, and encompasses both inflammatory and regulatory pathways.
Helicobacter Pylori Model
Our computational simulations using ODE modeling show a distinct time-dependent behavior in the three CD4+ T cell phenotypes (i.e., Th1, Th17 and iTreg) represented in the model during H. pylori infection. Whereas Th17 is crucial at an early stage of the infection, Th1 predominates over Th17 and is key for the chronicity of the infection in the gastric LP. Together with these responses, there is a regulatory T cell upregulation peaking at day 30 and being persistent over the infection. Experimentaly, stomachs of H. pylori infected mice have more histopathological lesions(Figure 3F, 3G) To determine and track the main responsible subsets triggering such lesions, sensitivity analysis (SA) methods were applied. Results showed how at the early stage of infection, the epithelial cell damage is mainly caused by the bacterium itself (Figure 2A). Interestingly, as the infection progresses, a trend towards Th1 cells triggering epithelial cell damage (Figure 2B) is observed. At the chronic phase of the infection, results showed a dramatic increase of Th1- and Th17-inducing epithelial cell damage (Figure 2C). Of note, SA performed in the deterministic model at day 60 post infection also showed how Th1 and Th17 in both LP and GLN were contributing to the formation and accumulation of damaged epithelial cells as well as M1 macrophage differentiation, whereas H. pylori exhibited no impact on such formation (Figure 8D).
The following is a list of archived COPASI Helicobacter Pylori computational model releases. Please click on individual releases for more details.
The primary MIEP team members responsible for maintaining this model are Adria Carbo, Mireia Pedragosa and Kate Wendelsdorf at the Virginia Bioinformatics Institute. Please contact them with any questions or comments. For the latest release, The model is available for download in CellDesigner xml format. We have tested that the model is compatible with Cell Designer 4.1. The following is the structure figure of the model, and by clicking on the figure you can navigate the model through a Google-Map-API-enabled CellPublisher user friendly interface.- Fourth, the latest, release on May 27th, 2012
- Third release on April 28th, 2012
- Second release on January 14th, 2011
- First release on September 15th, 2011
Modeling of immune responses towards H. pylori - Agent Based model
Modeling H. pylori using ENISI and Cell Designer Updated on December 29th 2012: The ENISI model has been progressed in the following two areas: Sensitivity Analysis and Cell Movement Modeling. To further characterize the immunological mechanisms underlying mucosal immune responses to H. pylori in a stochastic system, we used ABM based on parameter values derived from our ODE model. When probabilistic approaches are used, the complex immunological processes can be better represented. We adopted the ABM tool ENteric Immune Simulator (ENISI) developed by us and available here. In this case, a stochastic Agent Based Modeling approach has been used to better represent the biological system and add a complementary view on the cascade after infection. ABM adds randomness to the biological systems, which can help to better represent complex cellular responses and to take into account the individual behaviors of cells as well as the role spatiotemporal features. Thus, stochastic models can provide novel insights into the effect of cognate and non-cognate interactions, representing entire systems with a greater granularity and capturing cell-cell interactions. By simulating individual behaviors of agents, ABM better represents cross-linked, complex and nonlinear processes with multiple feedback loops and, provides a more comprehensive and interactive modeling of mucosal immune responses to H. pylori. The ability of ABM to encompass multiple scales of biological processes and incorporate spatiotemporal considerations, coupled with an intuitive modeling paradigm, underscores the added value of this modeling framework in translational systems immunology and immunoinformatics research. Given the complexity, nonlinearity and abundance of feedback loops in mucosal immune responses to H. pylori and to facilitate a better understanding of the mechanisms underlying such immune responses at the systems level, we constructed a SBML network model depicting the major effector and regulatory pathways evoked during H. pylori infection (Figure 1). Three different compartments are represented: gastric lumen, epithelium and lamina propria. Effector subsets are highlighted in red whereas regulatory subsets are highlighted in blue. Some updates on the HP ENISI model contain:
- New states of bacteria have been added which incorporates alive, dead and resting bacteria.
- The lifetime of each cell has been modified. Now each cell has a lifetime with normal distribution with a mean and standard deviation.
- We have changed the model parameters to adequate the model to different scenarios of experimental interest. Those scenarios can be accessed here.
- We have reduced some unnecessary states that were not needed in the system.
- We have created a generator application that creates the initial simulation files. This application has been updated with the additional initial states.
The model is available for download in CellDesigner xml format (right click the link or ctrl+left click for Mac machines to save the model source file). We have tested that the model is compatible with Cell Designer 4.2. The primary MIEP team members responsible for maintaining this model are Maksudul Alam, Adria Carbo and Yongguo Mei at the Virginia Bioinformatics Institute. Please contact them with any questions or comments. (Click on the image for a user-friendly interactive CellPublisher model.)
Highlights
- Our predictive model of immune responses towards Helicobacter pylori produced a series of hypotheses that were validated with immunology experiments.
- Simulation results show the induction of a Th17 response and a dominant Th1 response, together with a regulatory response characterized by high levels of mucosal Treg) cells.
- Sensitivity analyses sensitivity analysis predicted a crucial contribution of Th1 and Th17 effector responses as mediators of histopathological changes in the gastric mucosa during chronic stages of infection, which were experimentally validated in mice.
Abstract
T helper (Th) cells play a major role in the immune response and pathology at the gastric mucosa during Helicobacter pylori infection. There is a limited mechanistic understanding regarding the contributions of CD4+ T cell subsets to gastritis development during H. pylori colonization. We used two computational approaches: ordinary differential equation (ODE)-based and agent-based modeling (ABM) to study the mechanisms underlying cellular immune responses to H. pylori and how CD4+ T cell subsets influenced initiation, progression and outcome of disease. To calibrate the model, in vivo experimentation was performed by infecting C57BL/6 mice intragastrically with H. pylori and assaying immune cell subsets in the stomach and gastric lymph nodes (GLN) on days 0, 7, 14, 30 and 60 post-infection. Our computational model reproduced the dynamics of effector and regulatory pathways in the gastric lamina propria (LP) in silico. Simulation results show the induction of a Th17 response and a dominant Th1 response, together with a regulatory response characterized by high levels of mucosal Treg) cells. We also investigated the potential role of peroxisome proliferator-activated receptor γ (PPARγ) activation on the modulation of host responses to H. pylori by using loss-of-function approaches. Specifically, in silico results showed a predominance of Th1 and Th17 cells in the stomach of the cell-specific PPARγ knockout system when compared to the wild-type simulation. Spatio-temporal, object-oriented ABM approaches suggested similar dynamics in induction of host responses showing analogous T cell distributions to ODE modeling and facilitated tracking lesion formation. In addition, sensitivity analysis predicted a crucial contribution of Th1 and Th17 effector responses as mediators of histopathological changes in the gastric mucosa during chronic stages of infection, which were experimentally validated in mice. These integrated immunoinformatics approaches characterized the induction of mucosal effector and regulatory pathways controlled by PPARγ during H. pylori infection affecting disease outcomes.
Summary
Given the complexity, nonlinearity and abundance of feedback loops in mucosal immune responses to H. pylori and to facilitate a better understanding of the mechanisms underlying such immune responses at the systems level, we constructed a SBML network model depicting the major effector and regulatory pathways evoked during H. pylori infection (Figure 1).
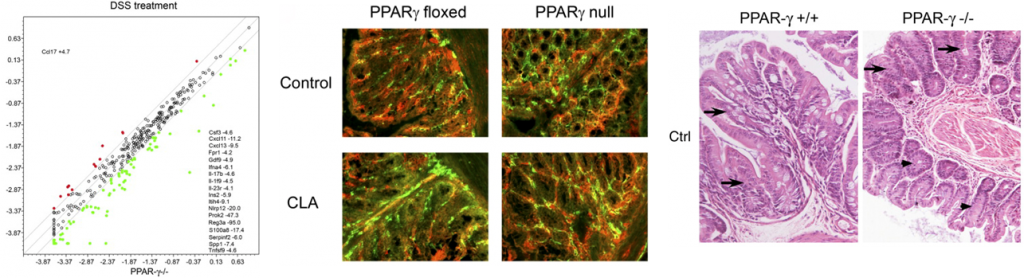
In line with our ODE-results, we observed in the ABM that in the GLN, Th1 cells peaked on day 30 post infection and remained at high levels with fairly constant values throughout the rest of the infection period (Figure 2A, 2G). Th17 responses were induced in the GLN and later detected in the LP, together with a Treg cell response that persisted over time in both gastric LP (Figure 2B, 2C) and GLN (Figure 2H, 2I). Regarding Th17 cells, these simulations depicted the immunoregulatory role of PPARg in the myeloid subset since we observed significant differences in enhanced Th17 responses in the myeloid cell-specific PPARg knockout model when compared to the wild-type. Th17 cell numbers were also significantly higher in the T cell-specific PPARg knockout model when compared to the wild-type model in both LP (Figure 2B and 2E) and the GLN (Figure 2H and 2K). T cell-specific PPARg deficiency significantly impaired the expansion of the iTreg cell compartment starting at day 30 and showed an oscillatory behavior and significant differences until day 60 in the gastric LP (Figure 2C and 2F).
We extended our modeling approaches to determine which are the main factors involved in gastric lesion development during H. pylori infection by using sensitivity analysis. Our results using ABM showed how at the early stage of infection (up to week 2 post-challenge), the epithelial cell damage is mainly caused by the bacterium (Figure 3A). Interestingly, we observed a trend towards Th1 and Th17 cells triggering epithelial cell damage starting 3 weeks post-infection. At the chronic phase of the infection our results showed a dominant role of Th1 and Th17 effector cells in inducing epithelial cell damage (Figure 3A). H. pylori induced epithelial cell damage throughout the infection. However, at a later infection stage, the induction of damaged epithelial cells by the effector Th1 and Th17 phenotypes overshadowed the effect of H. pylori itself. Of note, sensitivity analysis performed in the deterministic model at day 60 post-infection also showed similar results (Figure 3B). Going one step further from the model prediction, we hypothesized that the effector T cell response and not the bacterium itself is the main cause of epithelial cell damage during the chronic phase of H. pylori infection. Validating this hypothesis and using wild-type mice that were infected with H. pylori, immunophenotyping results showed a pronounced increase of IL-17A- (Figure 3C) and IFNg-producing cells (Figure 3D) in the gastric LP after 30 and 60 days post-infection. Metronidazole treatment, being an approach to eliminate the bacteria from the stomach at day 30, did not affect effector cytokine expression. These results suggested that effector T cell responses are implicated in lesion development during infection as showed in a cartoon model representation, highlighting the involvement of DC, T cells and macrophages on the formation of gastric lesions in the LP is shown in Figure 3E.
In summary, we combined computational modeling approaches and mouse challenge studies to investigate how CD4+ T cells and other immune cell subsets are distributed in the gut mucosa during H. pylori infection. Our model simulated T cell responses to H. pylori by using both platforms: ODE and ABM. Our modeling efforts predicted higher levels of effector responses in both the LP and the GLN when deleting PPARg, thus highlighting the role of PPARg activation as a potential mechanism for modulating CD4+ T cell responses during bacterial infection and positioning PPARg as a candidate for immunotherapeutics development. Future studies will more fully realize the potential of multiscale modeling to understand mucosal immunity.
References
a) Mei MH, R.; Zhang, X.; Bisset, K.; Eubank, S.; Hoops, S.; Marathe, M.; Bassaganya-Riera, J.; : ENISI Visual, an Agent-based Simulator for Modeling Gut Immunity. IEEE International Conference on Bioinformatics and Biomedicine (BIBM) 2012.
b) Mei Y, Carbo, A., Hontecillas, R., Bassaganya-Riera,J. : ENISI SDE: A novel web-based stochastic modeling tool for computational biology. 2013 IEEE International Conference on Bioinformatics and Biomedicine 2013.
c) Wendelsdorf KV, Alam M, Bassaganya-Riera J, Bisset K, Eubank S, Hontecillas R, Hoops S, Marathe M: ENteric Immunity SImulator: a tool for in silico study of gastroenteric infections. IEEE transactions on nanobioscience 2012, 11(3):273-288.
Data Figures
-
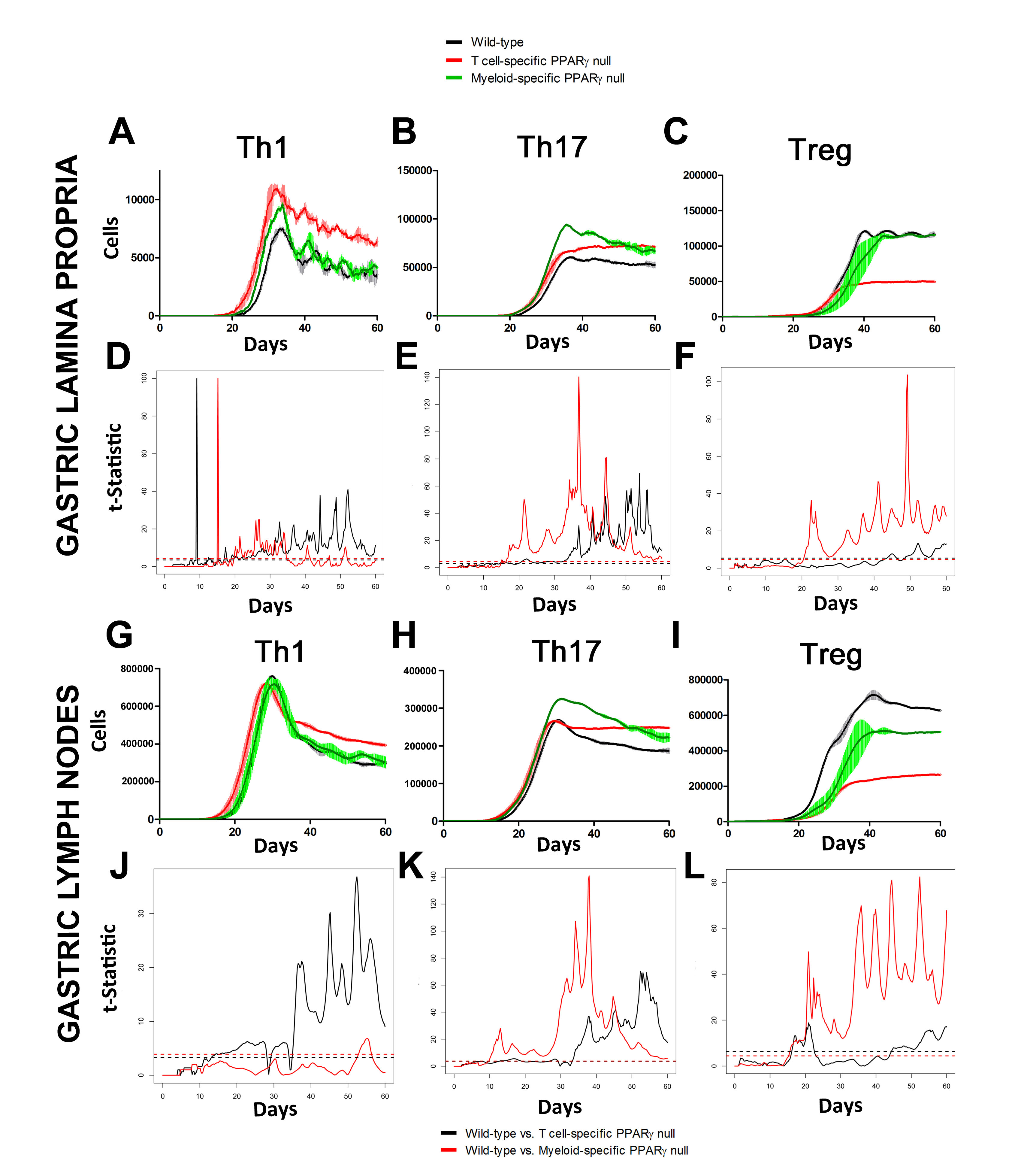 Figure 2. Enteric Immunity Simulator (ENISI) output results and assessment of the role of the Peroxisome Proliferator Activated Receptor g (PPARg) in both the myeloid and T cell subset modulated T cell responses after Helicobacter pylori infection in silico in the gastric lamina propria (LP) and gastric lymph nodes (GLN)
Figure 2. Enteric Immunity Simulator (ENISI) output results and assessment of the role of the Peroxisome Proliferator Activated Receptor g (PPARg) in both the myeloid and T cell subset modulated T cell responses after Helicobacter pylori infection in silico in the gastric lamina propria (LP) and gastric lymph nodes (GLN)
-
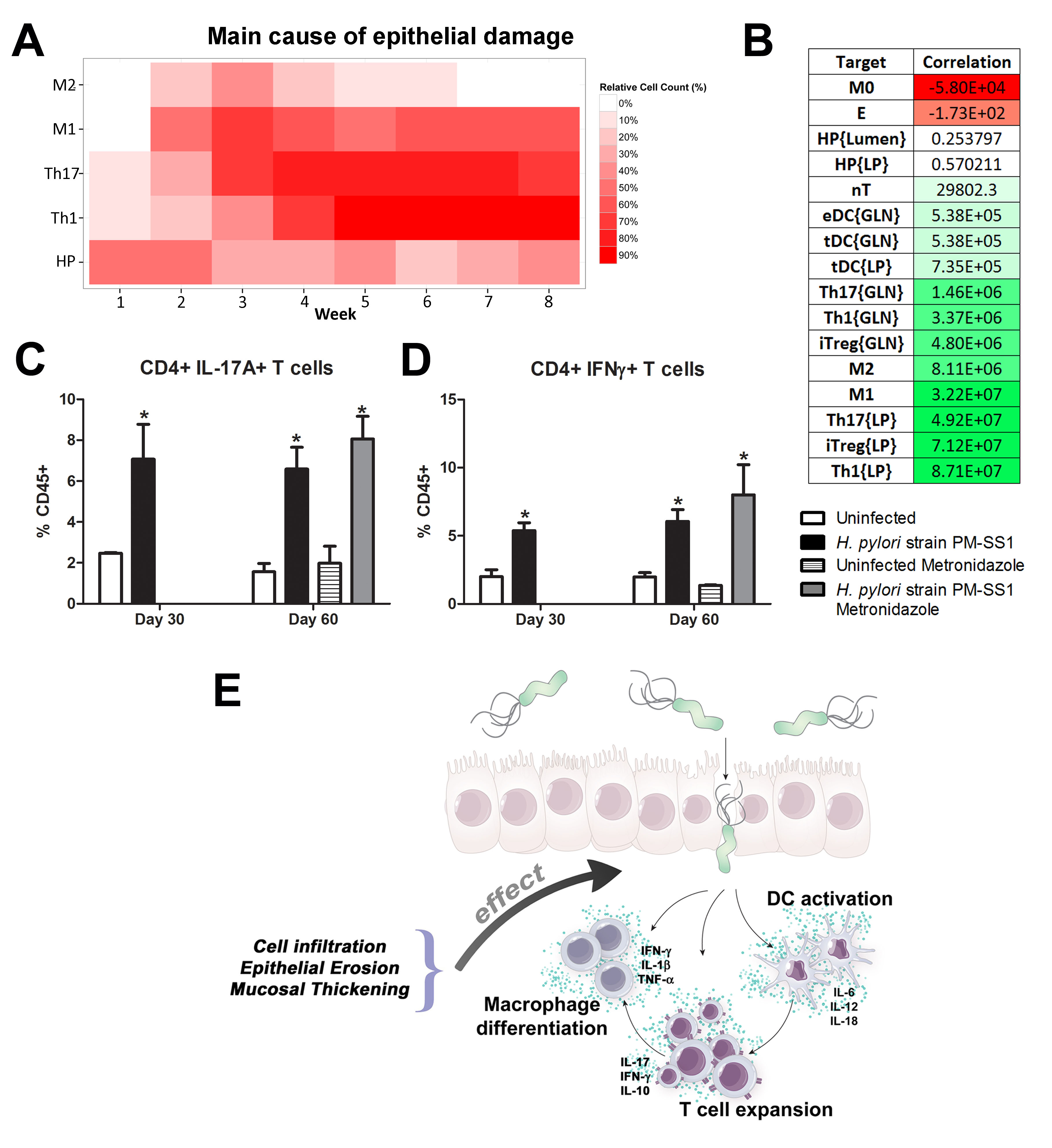 Figure 3. Sensitivity analysis of factors involved in gastric inflammatory lesion formation following Helicobacter pylori infection
Figure 3. Sensitivity analysis of factors involved in gastric inflammatory lesion formation following Helicobacter pylori infection
Highlights
- Pigs have greater anatomic, physiologic and immunological similarities to humans than mice, the main animal model used in biomedical research, and therefore have an enhanced translational value.
- The newly developed pig model of H. pylori infection closely mimics what has been reported in human clinical settings.
- Similar to humans, following infection of pigs with H. pylori there was an increase in CD4+ and CD8+ T cells, whereas CD8+ T cells remained unchanged in mouse and gerbil models, the main models used in biomedical research.
- The newly developed pig model allows NIMML to comprehensively and systematically investigate immune responses to H. pylori.
Abstract
Helicobacter pylori infection is the leading cause for peptic ulcer disease and gastric adenocarcinoma. Mucosal T cell responses play an important role in mediating H. pylori-related gastric immunopathology. While induced regulatory T (iTreg) cells are required for chronic colonization without disease, T helper (Th)1 effector responses are associated with lower bacterial loads at the expense of gastric pathology. Pigs were inoculated with either H. pylori strain SS1 or J99. Phenotypic and functional changes in peripheral blood mononuclear cell (PBMC) populations were monitored weekly, and mucosal immune responses and bacterial loads were assessed up to two months post-infection.
Both H. pylori strains elicited a Th1 response characterized by increased percentages of CD4+Tbet+ cells and elevated IFN-γ mRNA in PBMC. A subset of CD8+ T cells expressing Tbet and CD16 increased following infection. Moreover, a significant increase in perforin and granzyme mRNA expression was observed in PBMC of infected pigs indicating a predominant cytotoxic immune response. Infiltration of B cells, myeloid cells, T cells expressing Treg- and Th17-associated transcription factors, and cytotoxic T cells was found in the gastric lamina propria of both infected groups. Interestingly, based on bacterial reisolation data, strain SS1 showed greater capacity to colonize and/or persist in the gastric mucosa compared to strain J99.
This novel pig model of infection closely mimics human gastric pathology and presents a suitable avenue for studying effector and regulatory responses towards H. pylori described in humans.
Summary
To assess whether H. pylori infection affects the expansion of specific T cell subsets, we evaluated the expression of the main transcription factors involved in the regulation of CD4+ T cell phenotype: FOXP3 (iTreg and nTreg), Tbet (Th1) and RORγt (Th17). An elevated proportion of CD4+ T cells expressed the Th1-associated transcription factor Tbet in response to H. pylori (Fig. 1A). We observed a transient single peak at day 7 post-infection followed by a sustained increase in CD4+Tbet+ cells from day 28 to day 49 post-challenge in SS1-infected pigs. The same pattern was found in J99-infected pigs except that the response declined by day 49 post-challenge. The percentage of RORgt+CD4+ T cells was significantly different on days 21 post-infection for SS1 and days 28 and 49 post-infection in J99-infected pigs (Fig. 1C). There was a more notable decline in the expression of the Treg-associated transcription factor FOXP3 in CD4+ T cells from pigs infected with the strain SS1 on day 28 post-infection (Fig. 1D) which mirrored the increase in Tbet and coincided with increased transcripts of IFN-g mRNA in PBMC for both SS1 and J99-infected pigs (Fig. 1B). These results provided the first indication of a predominant Th1 response induced in our experimental model by H. pylori.
CD4+ T cells expressing the T cell lineage specific transcription factors (A) Tbet (Th1), (C) RORγt (TH17) and (D) FOXP3 (iTreg, nTreg) were detected by flow cytometry. IFN-γ mRNA levels in PBMC were measured by qRT-PCR (B). Symbols indicate statistical differences between either the H. pylori J99 (#) or SS1 (*) infected group to the control group and between both infected groups (&), n=8-9, mean±SEM, p≤0.05.
Furthermore, we demonstrate that H. pylori infection results in a significant increase of Tbet expressing cytotoxic CD8b+ T cells (Fig. 2A-B). The shift in Tbet expression was first detected on day 35 post-infection when on average 62% of cells had detectable amounts of this transcription factor. The percentage of CD8b+Tbet+ cells declined thereafter although towards the end of the study, on day 49 post-infection, there were still significant differences between infected and non-infected pigs. A closer analysis revealed the expansion of circulating CD8β+ T cells (Fig. 2C) and an increase of CD16 expression on those circulatingCTL (Fig. 2D) upon infection. Furthermore, CD3-CD8a+ NK cells were significantly increased in blood of SS1-infected pigs on day 35 post-infection (Fig. 2E).
Expression of Tbet was assessed in peripheral CTL (CD8β+) on day 42 post-infection (A) and over time (B). Representative flow cytometry dot blots for non infected and H. pylori J99 and SS1 infected pigs are presented. Numbers indicate percentage of positive cells within the single cell population (A). The number of (C) CTL (CD8β+) and (E) NK cells (CD3-CD8α+), were enumerated in PBMC over time. Numbers of cells per ml of blood were calculated by applying the percentage of immune cells obtained by flow cytometry to the concentration of cells in whole blood. The percentage of CD16 expressing CTL was assessed throughout the study (D). Gene expression levels of perforin (F), granzyme A (G) and granzyme B (H) in PBMC were analyzed over time. Symbols indicate statistical differences between either the H. pylori J99 (#) or SS1 (*) infected group to the control group and between both infected groups (&), n=8-9, mean±SEM, p≤0.05.
In concordance with the observed expansion of circulating cytotoxic T cells, we detected a significant upregulation in the expression of genes involved in the cytotoxic activity of CTL and NK cells, perforin, granzyme A and B (Fig. 2F-H). Overall our data suggest the initial induction of an IFN-g-producing Th1 response orchestrated by the transcription factor Tbet and executed by cytotoxic T cells.
Re-isolation of H. pylori from infected pigs was performed at the end of the study. Overall, H. pylori SS1 was recovered from the stomach of all pigs in that group, while H. pylori J99 could only be re-isolated from 8 out of 12 pigs. When looking at bacterial burden in different regions of the stomach we found that the percentage of re-isolation was consistently higher in the SS1-infected group than in the J99 group with the exception of the fundus-A sub-region which showed similar frequencies for both strains (Fig. 3B). Microscopic changes were present in the stomach of both infected groups and were characterized by significant expansion and development of organized lymphoid aggregates and diffuse leukocytic infiltration. Both strains of H. pylori induced organized lymphoid tissue in the stomach mucosa, which was more predominant in the cardiac region (Fig. 3A).
Representative images were taken from hematoxylin and eosin stained specimens collected from the stomach region cardiac B (CB) and pyloric A (PA) of non infected and infected pigs at 10× magnification (A). H. pylori J99 and SS1 were re-isolated from 6 stomach locations at 2 months post-infection. Re-isolation data is expressed as percentage (B).
In summary, our findings that H. pylori elicits Th1 and CTL responses in a novel pig model correlate well with its role as a facultative intracellular pathogen. Clinical and in vitro studies with human cells provide increasing evidence that cytotoxic immune responses play a crucial role in H. pylori pathogenesis. Our data demonstrates for the first time an increase in circulating CTL and NK cells peaking on day 35 post-infection which coincides with increased IFN-γ gene expression.
The exact role of CD8+ T cells and whether they contribute to the depletion of H. pylori from the gastric mucosa deserves further investigation. Findings from re-isolation and histopathology suggest that CD8+ T cell responses elicited upon infection might be ineffective in the elimination of bacteria but rather contribute to tissue damage. Furthermore, the infiltration of regulatory cells found in the stomach at least partially counteracts proinflammatory responses and contribute to bacterial persistence.
Here, we present the first pig model of H. pylori infection that corroborates in an experimental setting that the predominant Th1 response induced by the bacterium leads to the expansion of cytotoxic cells, including CTL and NK cells. The hallmark of the immune response to H. pylori in humans is the infiltration of Treg cells, neutrophils and Th1 cells. We have been able to reproduce these findings in our pig model, showing infiltration of myeloid cells and FOXP3+ T cells suggesting the presence of Tregs in the gastric mucosa. Furthermore, our model shows a strong systemic Th1 response followed by cytotoxic T cell responses. Similar to H. pylori mediated chronic gastritis in humans, bacteria are able to persist in the pig stomach but at the expense of lesion development. While the role of CD8+ T cells in mouse models of H. pylori has only been studied using immunodeficient mice lacking CD4+ T cells, our pig model provides a more suitable in vivo system to study cytotoxic immune responses towards H. pylori observed in humans and an ideal setting for testing new therapeutic approaches.
Link to the raw data can be found here.
Link to the publication can be found here.
Link to the press release can be found here.
Nutritional Immunology and Molecular Medicine Preclinical Studies
The NIMML pre-clinical studies have an added translational value by the incorporation of pig models of infectious and immune-mediated diseases, including inflammatory bowel disease (IBD) and infections caused by enteric or respiratory pathogens. As opposed to mice, pigs lend a physiological composition more comparable to humans, specifically the mucosal immune system, of the pig more closely resembles that of human and are thus an ideal model for studying the complexity of the human immune system and how it responds to inflammation, infection, and injury. Preclinical studies using pigs lay the groundwork for an informed design of human clinical trials.
Our Facilities and Animals
Our AAALAC-accredited swine facility consists of 16 separate experimental housing rooms with capacities of 8 pigs each. A fully equipped surgical room is located in the building for onsite collection of specimens. Supervisors, students and animal technicians are able to provide superior husbandry for pigs spanning all ages of the life cycle. The majority of our projects use neonatal to weaned pigs. Gnobiotic swine litters are also available. Procedures routinely performed in the swine studies include venipuncture, injections, immunizations, and intubation. All procedures and experiments are approved by the Institutional Animal Care and Use Committee (IACUC). Our facility is AAALAC-accredited and can be used for biosafety level 1 (ABSL1) and ABSL2 studies. Moreover, the studies can be run as Good Laboratory Practices (GLP), thereby fulfilling the requirements of regulatory agencies for IND packages. Active monitoring post-challenge is performed to ensure the well-being of the animals as well as provide an assessment of the disease activity progression throughout the duration of the experiment. Peripheral blood collection is performed on a regular basis as a means of analyzing the time course of an infection. Execution of specific expected goals is met with superior efficiency in a timely manner while maintaining detailed attention to our animals and data.
Neonatal Model
Extensive research has shown the similarities in human neonates compared to swine neonates regarding neonatal nutrition and digestion as well as the immunoregulatory system thus rendering this type of model preferable over mice or other rodent species for pre-clinical trials. Changes in neonatal pigs represent rapid intestinal growth and functions surrounding nutrient absorption and immune functions. When studying the effects of diseases such as Enteroaggregative E. coli or rotovirus which are particular to neonates, the demand to use a model that is more representative of the chronic infections is necessary. Swine have a gestation length of 113-115 days and synchronization techniques allow for timed parturition and project set-up. Piglets used for neonatal projects are removed from the mother immediately at birth to be placed into highly regulated state of the art facilities uniquely designed for the piglets. Our facilities are able to provide specialty care for early weaned neonates and maintain projects for long term. Piglets have twenty-four hour access to milk by means of our custom designed bottles which are continuously checked by trained personnel. Piglets are housed in their own incubator for intensive monitoring and thermoregulation control as well as individual feeding. Specialty care throughout the duration of their lives is under strict control of IACUC approved protocols.
Selected Publications
- Differential requirements for proliferation of CD4+ and gammadelta+ T cells to spirochetal antigens. Hontecillas R, Bassaganya-Riera J. Cell Immunol. 2003 Jul;224(1):38-46. [PubMed]
- Conjugated linoleic acid ameliorates viral infectivity in a pig model of virally induced immunosuppression. Bassaganya-Riera J, Pogranichniy RM, Jobgen SC, Halbur PG, Yoon KJ, O’Shea M, Mohede I, Hontecillas R. J Nutr. 2003 Oct;133(10):3204-]14. [PubMed]
- Dietary conjugated linoleic acid modulates phenotype and effector functions of porcine CD8(+) lymphocytes. Bassaganya-Riera J, Hontecillas R, Zimmerman DR, Wannemuehler MJ. J Nutr. 2001 Sep;131(9):2370-7. [PubMed]
- Effects of dietary conjugated linoleic acid in nursery pigs of dirty and clean environments on growth, empty body composition, and immune competence. Bassaganya-Riera J, Hontecillas-Magarzo R, Bregendahl K, Wannemuehler MJ, Zimmerman DR. J Anim Sci. 2001 Mar;79(3):714-21. [PubMed]
Gut Inflammation Studies
Inflammatory bowel disease (IBD) is a chronic reoccurring inflammatory illness affecting millions of people worldwide. IBD has two clinical manifestations: Crohn’s disease and ulcerative colitis. The NIMML developed an acute model of colitis in pigs by intragastric administration of dextran sulfate sodium (DSS) which causes epithelial erosion and subsequent leucocytic infiltration. Another chemical irritant which induces a slightly more severe IBD is 2,4,6-trinitrobenzene sulphonic acid (TNBS) which induces T helper 1 (Th1) and Th17 responses in the colonic mucosa. The NIMML can implement pig projects of DSS, TNBS or bacterial-induced colitis to measure the anti-inflammatory efficacy of new IBD treatments. Gut Inflammation Studies Provide Data for:
- Biomarker discovery
- Therapeutic target discovery
- Bioinformatics analyses
- Immunological assays
- Histopathology services
Selected Publications
- CLA and n-3 PUFA differentially modulate clinical activity and colonic PPAR-responsive gene expression in a pig model of experimental IBD. Bassaganya-Riera J, Hontecillas R. Clin Nutr. 2006 Jun;25(3):454-65. Epub 2006 May 15.
- CD4+ T-cell responses and distribution at the colonic mucosa during Brachyspira hyodysenteriae-induced colitis in pigs. Hontecillas R, Bassaganya-Riera J, Wilson J, Hutto DL, Wannemuehler MJ. Immunology. 2005 May;115(1):127-35.
- Nutritional regulation of porcine bacterial-induced colitis by conjugated linoleic acid. Hontecillas R, Wannemeulher MJ, Zimmerman DR, Hutto DL, Wilson JH, Ahn DU, Bassaganya-Riera J. J Nutr. 2002 Jul;132(7):2019-27.
Infectious Disease Studies: Gastrointestinal Pathogens
- The NIMML has a primary focus on studying the mucosal immune responses to gastrointestinal and respiratory pathogens. Likewise, pre-clinical studies involving infectious disease also provide valuable mechanistic insights on efficacy and safety of vaccines and immune therapeutics. Our projects produce extensive results which can be implemented directly into our modeling efforts for calibration and fitting of model parameters. This approach allows us to begin inferring methods which are more predictive in studying a complex biological system. Risks for infection caused by gastrointestinal pathogens increases for people who travel internationally, live impoverished under malnourished diets, are immunocompromised, or consume the pathogen through contaminated food resulting in a food borne illness. Initial infection usually leads to gastrointestinal discomfort and subsequent diarrhea. When challenged with these pathogens and a persistent chronic illness occurs, painful ulcers form in the gastric (H. pylori) and intestinal tracts due to prolonged inflammation and the necrosis of tissue. Pre-clinical models of these pathogens are vital in the development of therapeutics which will translate into the development human clinical trials. The primary gastrointestinal infectious diseases studied by NIMML under the MIEP program include infections caused by Helicobacter pylori, Clostridium difficile, and Enteroaggregative Escherichia coli (EAEC) although other models can be easily developed.
- Specific strains used for infectious disease models:
- Enteroaggregative Escherichia coli(EAEC)
- JM221 Strain
- 042 Strain
Helicobacter pylori
- European 26695 strain
- African J99 strain
- SS1 strain
Clostridium difficile
- UVA13 strain
- VPI 11186 strain
- 10463 strain
- Antigen-specific proliferation of porcine CD8alphaalpha cells to an extracellular bacterial pathogen. Waters WR, Hontecillas R, Sacco RE, Zuckermann FA, Harkins KR, Bassaganya-Riera J, Wannemuehler MJ. Immunology. 2000 Nov;101(3):333-41.
- Long-term influence of lipid nutrition on the induction of CD8(+) responses to viral or bacterial antigens. Bassaganya-Riera J, Hontecillas R, Zimmerman DR, Wannemuehler MJ. Vaccine. 2002 Jan 31;20(9-10):1435-44.
The NIMML is a fully integrated translational effort with capabilities of performing a wide variety of validated immunological assays in pre-clinical and clinical settings as GLP or non-GLP. Experimental variation is tightly controlled through the refinement of protocols for techniques thus generating reliable quality data and our laboratory information management and integration systems.
Selected Publications
Infectious Disease Studies: Respiratory Pathogens
The seasonal flu is a highly contagious illness which affects between 5-20% of people annually in the US. Due to the mutagenic characteristic of the orthomyxovirus, new strains appear each year amounting more than 200,000 hospitalizations due to infection and respiratory complications. The NIMML primarily studies influenza A virus. This genus has the ability to cause detrimental pandemics in a short period of time since it can be transmitted among different species. Specific strains in the NIMML include: H1N1 strain A/Pureto Rico/8/34 (PRS), H3N2 strain A/Udorn/72, and the H1N1 strain A/California/07/2009.
Our projects provide reliable data for:
- Clinical weight loss
- Viral disease progression
- Lung histology and immunohistochemistry
- Immunological Assays and flow cytometry
In addition, the NIMML has received IACUC approval to conduct LD50 studies for influenza projects.
Selected Publications
- Arachidonic acid and docosahexaenoic acid-enriched formulas modulate antigen-specific T cell responses to influenza virus in neonatal piglets. Bassaganya-Riera J, Guri AJ, Noble AM, Reynolds KA, King J, Wood CM, Ashby M, Rai D, Hontecillas R. Am J Clin Nutr. 2007 Mar;85(3):824-36.
- Impact of immunizations with porcine reproductive and respiratory syndrome virus on lymphoproliferative recall responses of CD8+ T cells. Bassaganya-Riera J, Thacker BJ, Yu S, Strait E, Wannemuehler MJ, Thacker EL. Viral Immunol. 2004;17(1):25-37.
Swine as a Translational Model
The NIMML has the ability to focus on diseases on a multitude of levels. Our projects begin using mouse models and as the data becomes more comprehensive, pigs provide the intermediate step prior to clinical trials. Our pig models have provided data that is critical in understanding the immune response to colitis, IBD, enteric pathogens, and respiratory infections. We continue to use the pig model to facilitate the development of projects which can assist in computational modeling components of the laboratory as well as potentiate the translation of research into human patients. Our streamlined facilities allow us to work with collaborators to collect data and allow for personnel to focus on their specializations. By using students trained in animal sciences and other degrees which provide education encompassing animal models for scientific research, our team is able to work together to make novel advancements in a broad range of scientific topics with efficiency and accuracy.
Preclinical services
The Nutritional Immunology and Molecular Medicine Laboratory offers a broad array of preclinical services in mouse, rats, hamsters and pigs for product testing, mechanism of action validation studies, and hypothesis–driven and hypothesis-generating in vivo experimentation. All our preclinical animal models are set up in Virginia Tech animal facilities and are approved by the Virginia Tech Institutional Animal Care and Use Committee (IACUC). The mice are housed in a state-of-art facility that can accommodate large projects as well as both animal biosafety level (ABL)-1 and ABL-2 experiments. These services will be provided as a part of a sponsored research agreement with Virginia Tech.
Mouse Models
Colitis (DSS, adoptive transfer of CD4+ T cells, IL-10 ko, TNBS, bacterial induced) Influenza infection Clostridium difficile infection Enteroaggregative Escherichia coli infection Helicobacter pylori infection Colorectal cancer Type 2 diabetes and obesity (db/db, diet-induced obesity, KK-Ay, ob/ob) Lupus Type 1 diabetes (NOD, STZ) Atherosclerosis (ApoE)
Pig Models
Colitis (Bacterial-induced, DSS, TNBS) Influenza infection Helicobacter pylori infection Neonatal models Radiation models
Rat Models
Type 1 diabetes Type 2 diabetes Safety and toxicity assessment PK/PD, ADME
Preclinical models for Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is characterized by two major clinical manifestations: Crohn’s disease (CD) and ulcerative colitis (UC). CD can affect any regions of the gastrointestinal (GI) tract from mouth to anus. In contrast, UC causes acute colonic inflammation. The NIMML offers expertise and services in mouse models of IBD such as trinitrobenzene sulfonic acid (TNBS), dextran sodium sulfate (DSS)-induced colitis, pan-enteritis due to the deficiency of interleukin-10 (IL-10 KO) and CD4+ T cell-induced colitis in adoptive transfer models. Whereas TNBS and DSS-induced colitis represent the more acute and faster induction of colonic tissue damage, the IL-10 KO and the CD4+ T cell induced colitis represent chronic models of IBD. We have also developed a model of inflammation-driven colorectal cancer (CRC) following treatment of mice with azoxymethane and DSS. The NIMML provides expertise and resources in these four preclinical models with more than 10 years of experience and results published in top gastroenterology journals. NIMML can provide data on
- Immunological changes at the gut mucosa and systemically
- Gut histological and immunohistochemical changes
- Transcriptomic analyses (RNAseq, ChipSep, miRNA)
- Pre-clinical efficacy, tolerability and weight loss
- Cellular and molecular profiling
Preclinical models for Infectious diseases: Respiratory pathogens
The NIMML has been working on Influenza models with several strains, being able to develop lung damage (i.e., epithelial necrosis, leucocyte infiltration), weight loss 10-20% and clinical disease. The virus strains we are using in mouse and pig challenge studies include:
- H1N1 strain A/Puerto Rico/8/34 (PR8)
- H3N2 strain A/Udorn/72
- H1N1 strain A/California/07/2009
- Viral disease progression, weight loss
- Histology and immunohistochemistry
- Vaccine and therapeutic efficacy
- Immunological assays and flow cytometry
- Viral titers
- LD50 studies
Preclinical models of Infectious diseases: Gastrointestinal pathogens
Yearly outbreaks of several pathotypes of E. coli, Salmonella, and other enteric pathogens incur medical costs, lost productivity and even premature death, with total expenses exceeding $6.9 billion per year. A closer comprehension of the mechanisms of action underlying immune responses to enteric pathogens will lead to the development of more efficacious vaccines and immunotherapeutics. Through the Modeling Immunity to Enteric Pathogens program, NIMML has build capabilities in modeling gut enteric infections. NIMML can reproduce and validate the following infectious disease models:
- Enteroaggregative Escherichia coli(EAEC)
- JM221 strain
- 042 strain
Helicobacter pylori
- European 26695 strain
- African J99 strain
- SS1 strain
Clostridium difficile
- UVA13 strain
- VPI 11186 strain
- 10463 strain
Preclinical models to validate computational model approaches
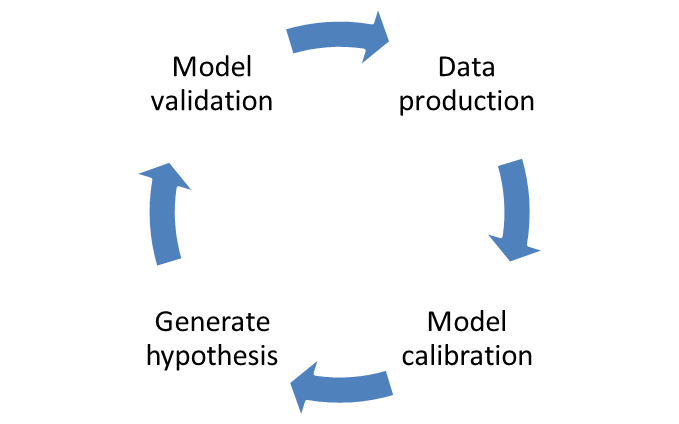
The NIMML works in the interface between the experimental and modeling/simulation approaches to study the host responses to gut and respiratory pathogens. The modeling process requires strong crosstalk with the experimental side: model calibration and validation. Each model needs to be calibrated with experimental data to ensure correct fitting and the correct assignment on numerical values for the parameters in differential equations. Afterwards, when the model is fully calibrated with experimental data, a new window to experimental design is opened: model validation. Resulting in model-aided, hypothesis-driven experimental validation, positioning computational modeling as a effective and advanced tool for immunology research and discovery. The NIMML has expertise in developing new designs depending on the needs of the customer. We are currently working in several validation studies for mathematical models under the MIEP program and we are flexible to design new models with creation of knockouts with the zinc finger technology. This validation approach will accelerate the whole process into a final publication, showing how computational method can validate biological processes.
Selected References
- Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease Bassaganya-Riera J, Reynolds K, Martino-Catt S, Cui Y, Hennighausen L, Gonzalez F, Rohrer J, Benninghoff AU, Hontecillas R. Gastroenterology. 2004 Sep;127(3):777-91. [PubMed]
- Peroxisome proliferator-activated receptor gamma is required for regulatory CD4+ T cell-mediated protection against colitis Hontecillas R, Bassaganya-Riera J. J Immunol. 2007 Mar 1;178(5):2940-9. [PubMed]
- T cell PPARγ is required for the anti-inflammatory efficacy of abscisic acid against experimental IBD. Guri AJ, Evans NP, Hontecillas R, Bassaganya-Riera J. J Nutr Biochem. 2011 Sep;22(9):812-9. Epub 2010 Dec 15. [PubMed]
- Immunoregulatory actions of epithelial cell PPAR gamma at the colonic mucosa of mice with experimental inflammatory bowel disease. Mohapatra SK, Guri AJ, Climent M, Vives C, Carbo A, Horne WT, Hontecillas R, Bassaganya-Riera J. PLoS One. 2010 Apr 20;5(4):e10215. [PubMed]
- Immunoregulatory mechanisms of macrophage PPAR-γ in mice with experimental inflammatory bowel disease. Hontecillas R, Horne WT, Climent M, Guri AJ, Evans C, Zhang Y, Sobral BW, Bassaganya-Riera J. Mucosal Immunol. 2011 May;4(3):304-13. Epub 2010 Nov 10. [PubMed]
- The role of T cell PPAR gamma in mice with experimental inflammatory bowel disease. Guri AJ, Mohapatra SK, Horne WT 2nd, Hontecillas R, Bassaganya-Riera J. BMC Gastroenterol. 2010 Jun 10;10:60. [PubMed] 7. Dietary α-eleostearic acid ameliorates experimental inflammatory bowel disease in mice by activating peroxisome proliferator-activated receptor-γ. Lewis SN, Brannan L, Guri AJ, Lu P, Hontecillas R, Bassaganya-Riera J, Bevan DR. PLoS One. 2011;6(8):e24031. Epub 2011 Aug 31. [PubMed]


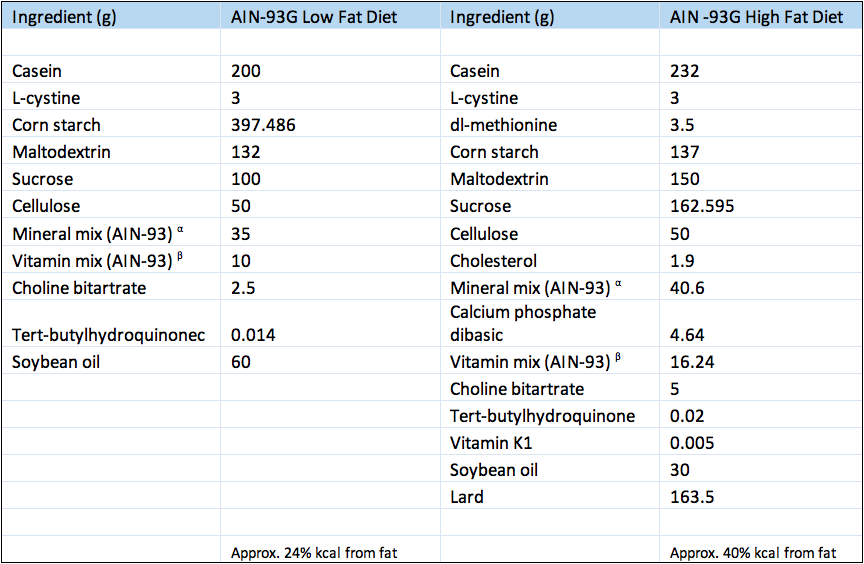 Composition of experimental diets.
Composition of experimental diets.
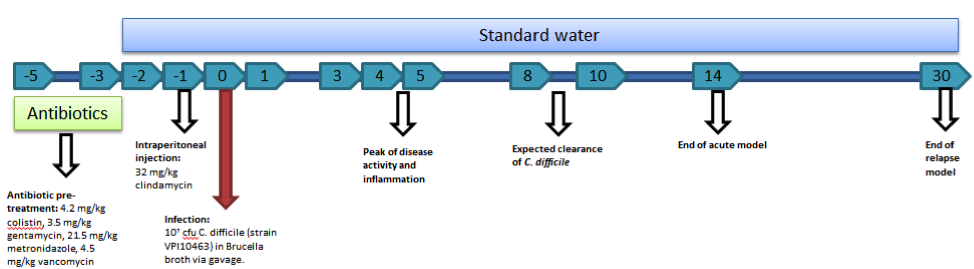 Mouse model of C. difficile infection creates colonic inflammation and symptoms of gastrointestinal disease.
Mouse model of C. difficile infection creates colonic inflammation and symptoms of gastrointestinal disease.
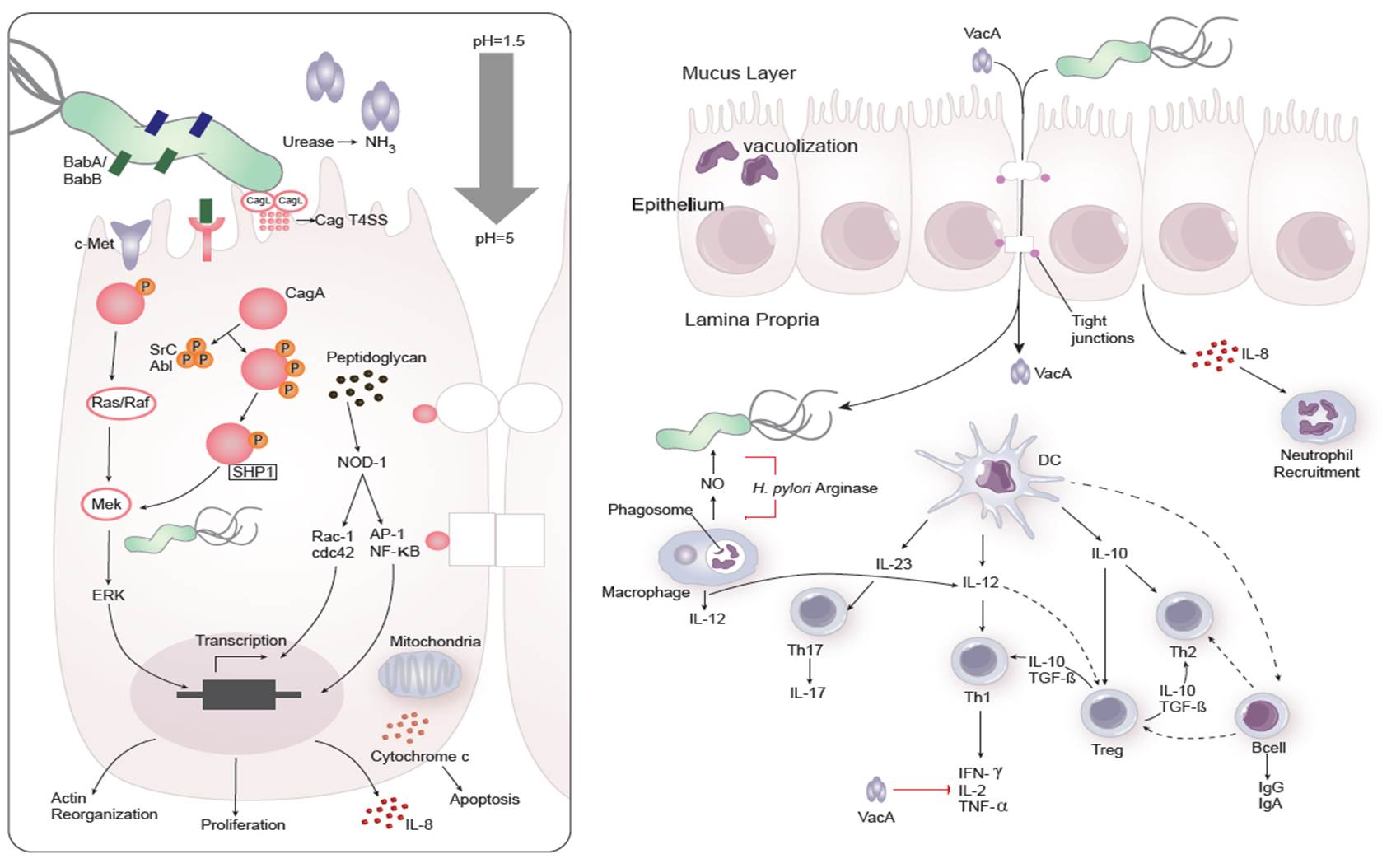 Figure 1. Cellular and molecular pathogenesis of Helicobacter pylori infection
Figure 1. Cellular and molecular pathogenesis of Helicobacter pylori infection
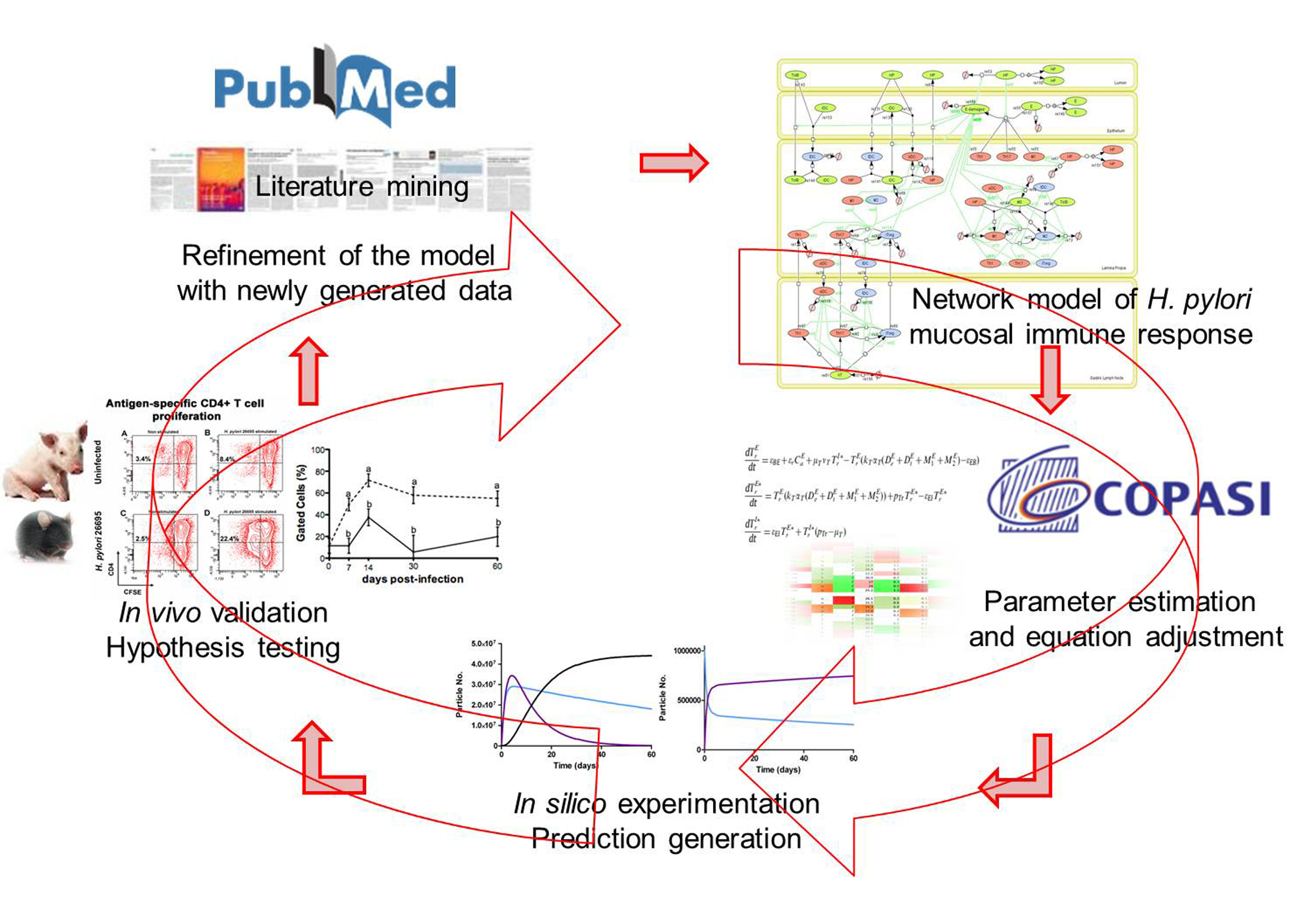 Figure 2. Integration of computational approaches and experimental data.
Figure 2. Integration of computational approaches and experimental data.
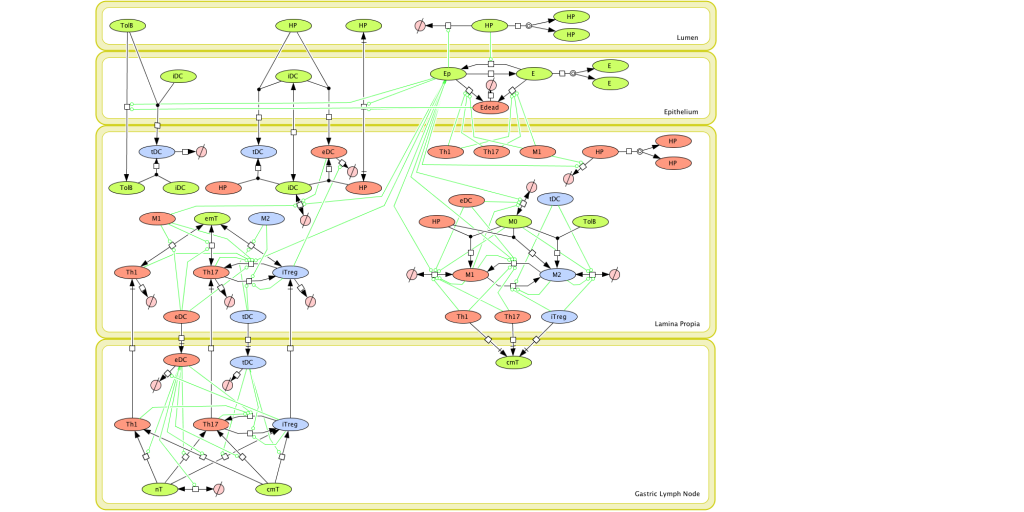 Figure 1. Computational model of the mucosal immune responses to Helicobacter pylori.
Figure 1. Computational model of the mucosal immune responses to Helicobacter pylori.
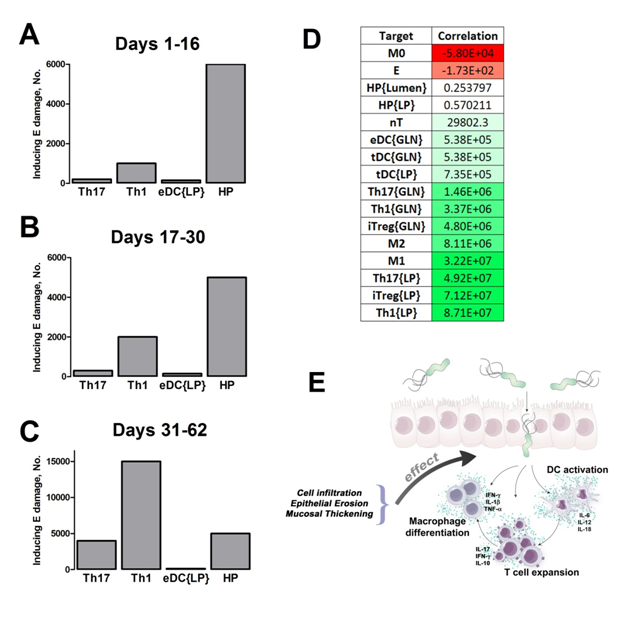 Figure 2. Sensitivity analysis on gastric inflammation lesion formation following Helicobacter pylori infection in silico.
Figure 2. Sensitivity analysis on gastric inflammation lesion formation following Helicobacter pylori infection in silico.
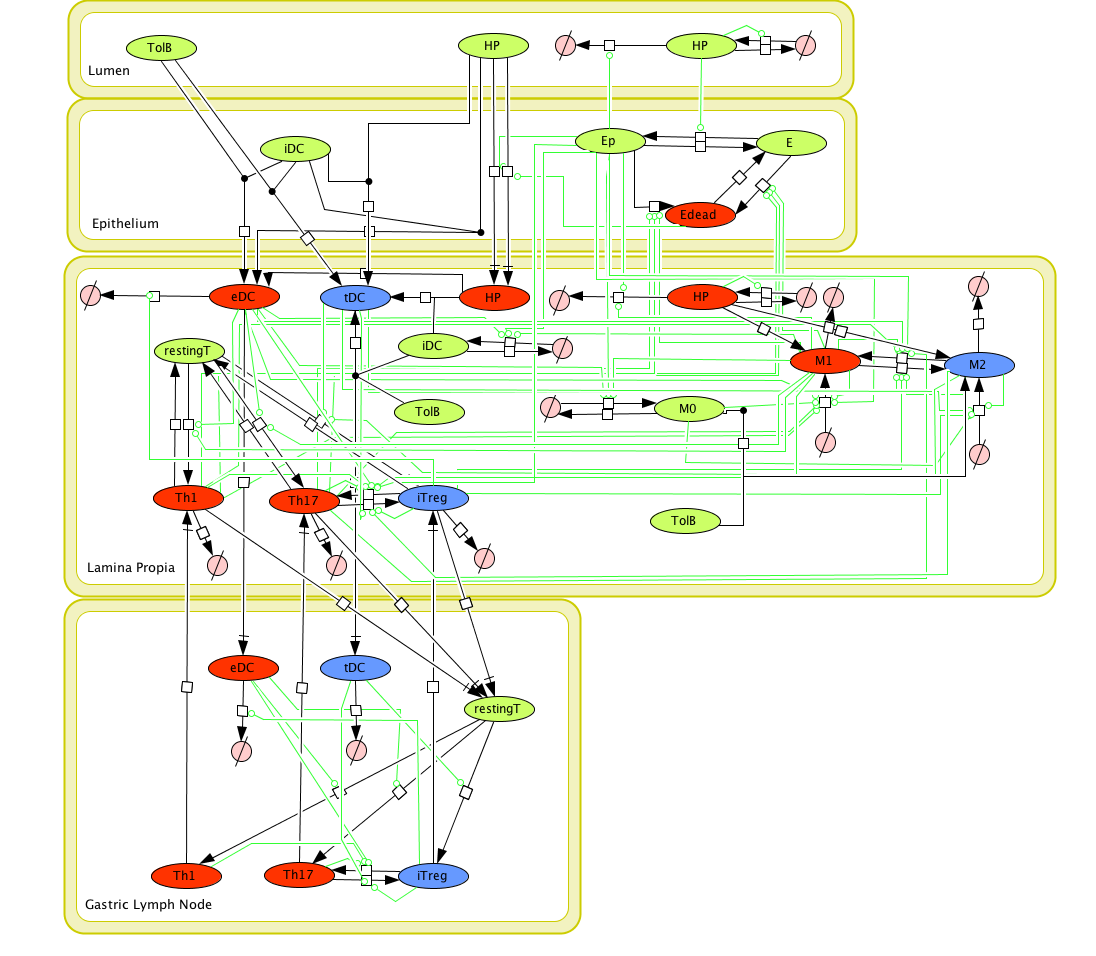 Figure 1. Cell Designer image: ENISI model of H. pylori
Figure 1. Cell Designer image: ENISI model of H. pylori
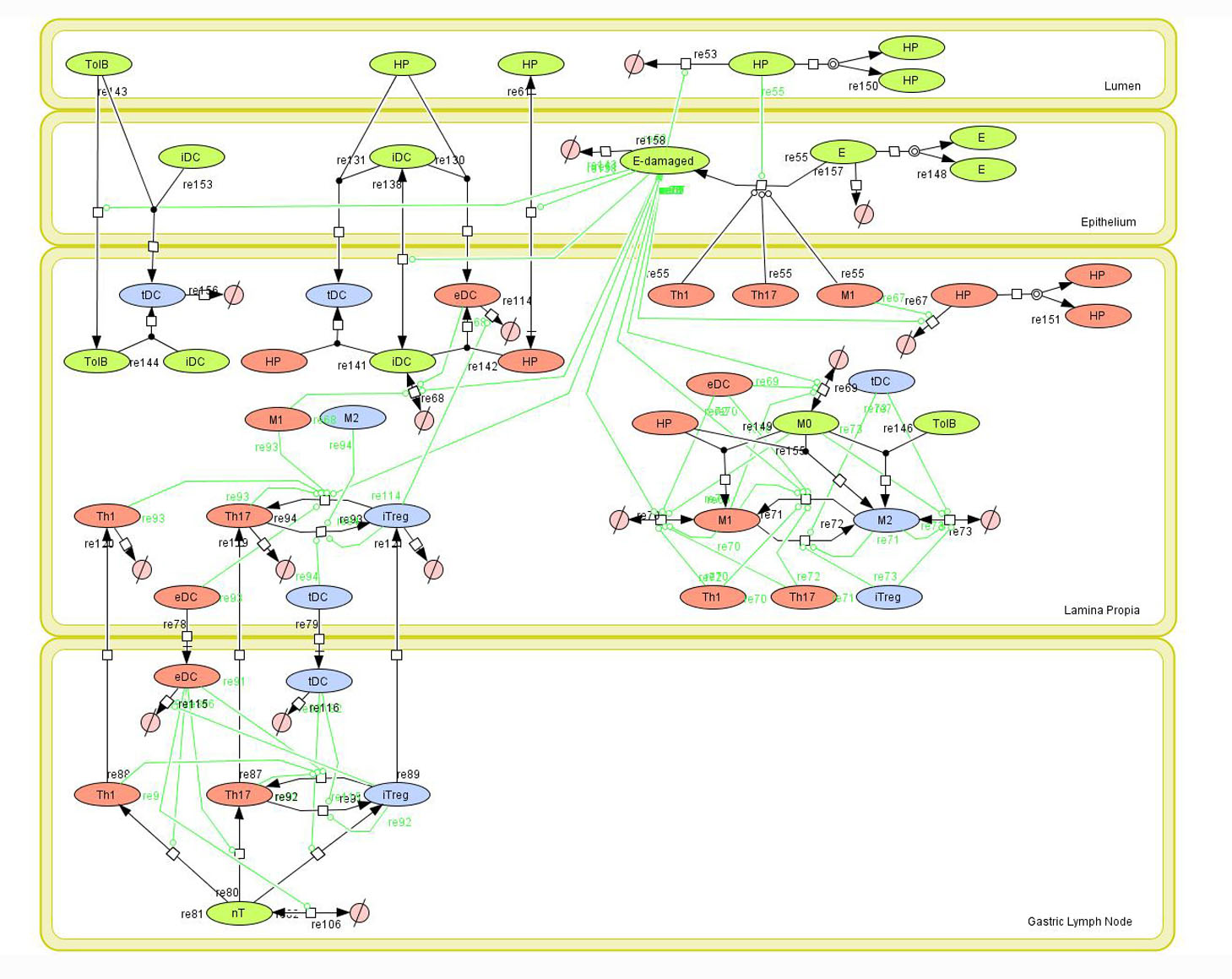 Figure 1. Network model of the mucosal immune responses during Helicobacter pylori infection. Systems Biology Markup
Figure 1. Network model of the mucosal immune responses during Helicobacter pylori infection. Systems Biology Markup
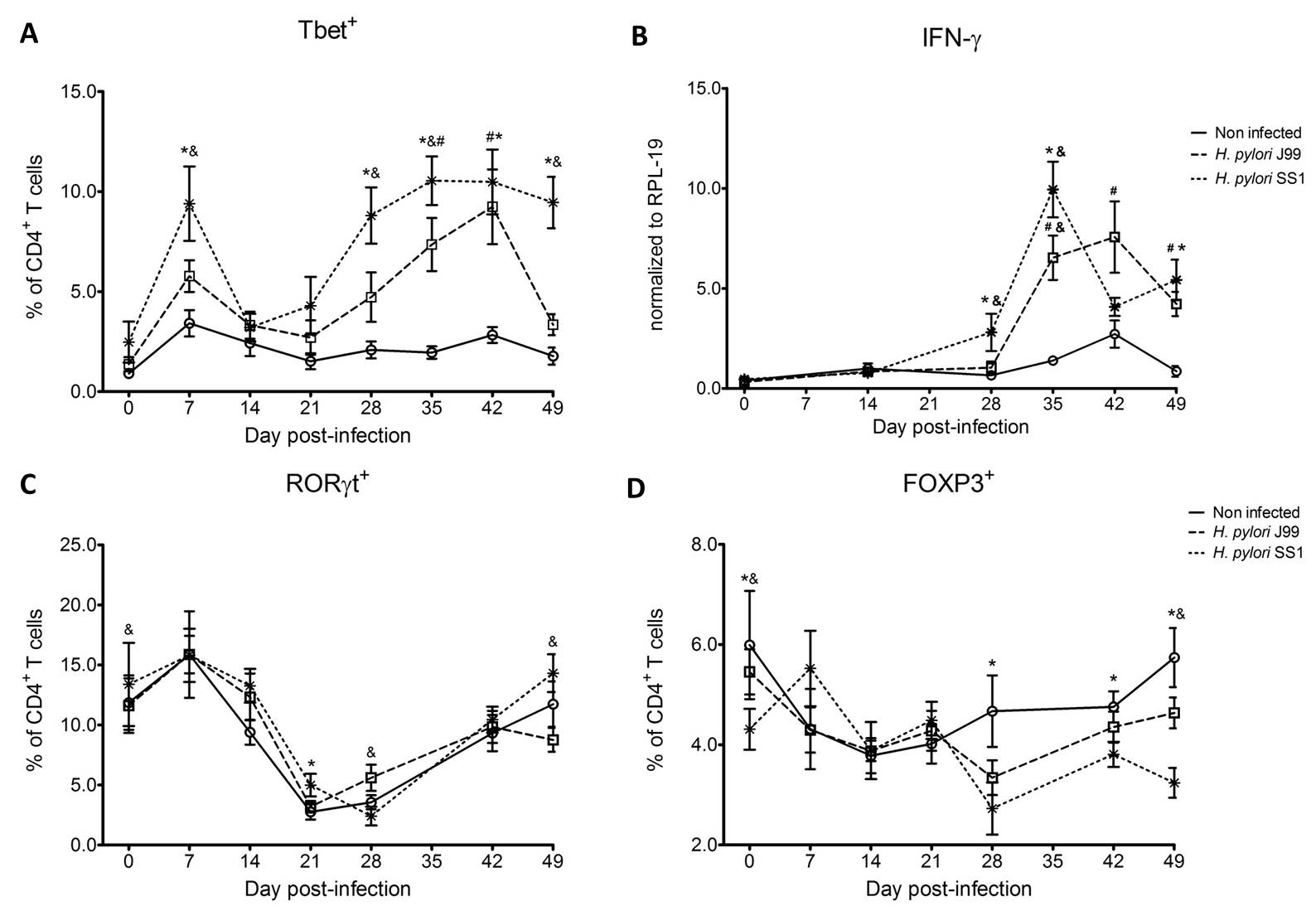 Figure 1. Increase in circulating Th1 cells and IFN-γ expression upon H. pylori infection.
Figure 1. Increase in circulating Th1 cells and IFN-γ expression upon H. pylori infection.
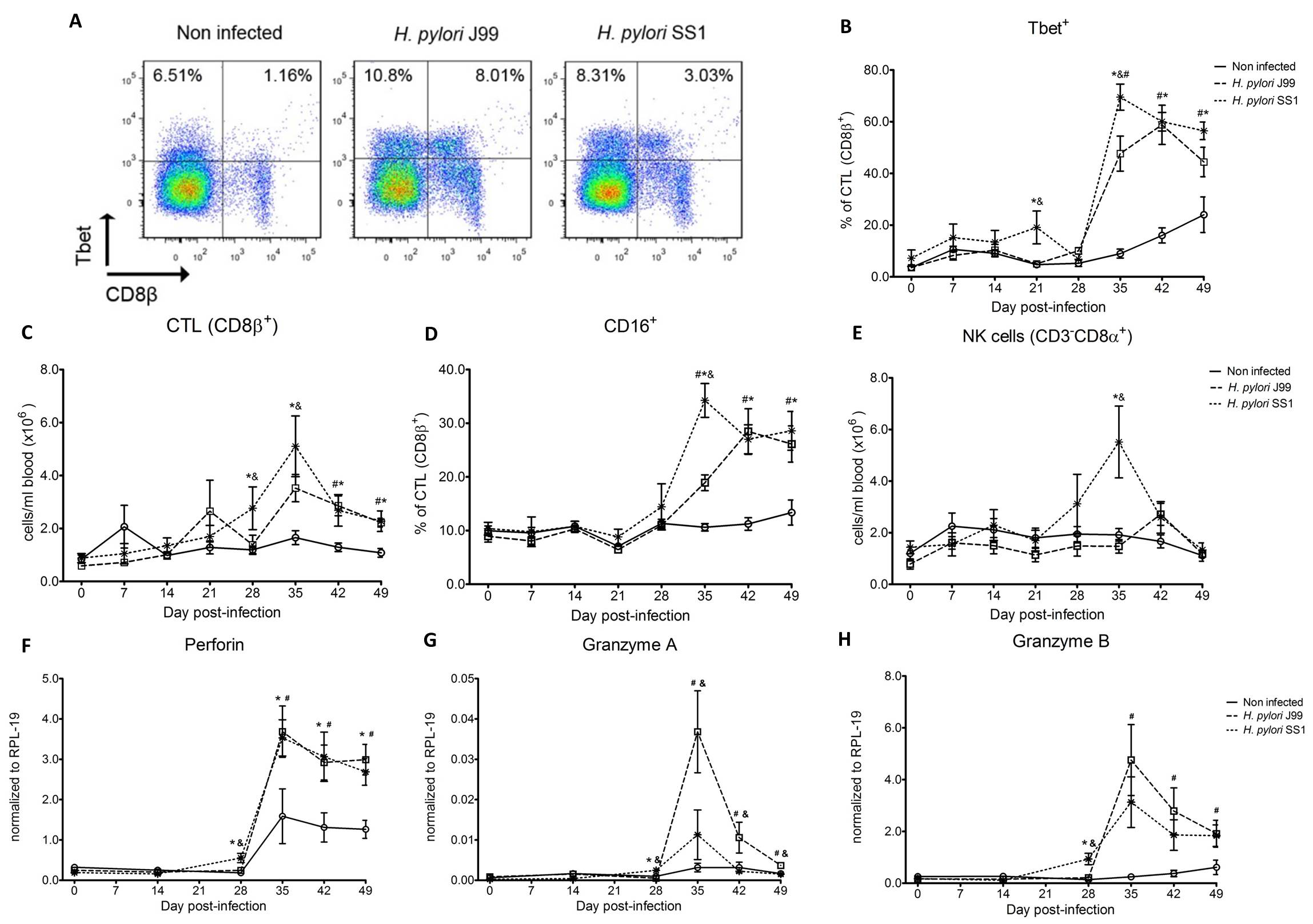 Figure 2. Increased circulating and functional cytotoxic cells upon H. pylori infection.
Figure 2. Increased circulating and functional cytotoxic cells upon H. pylori infection.
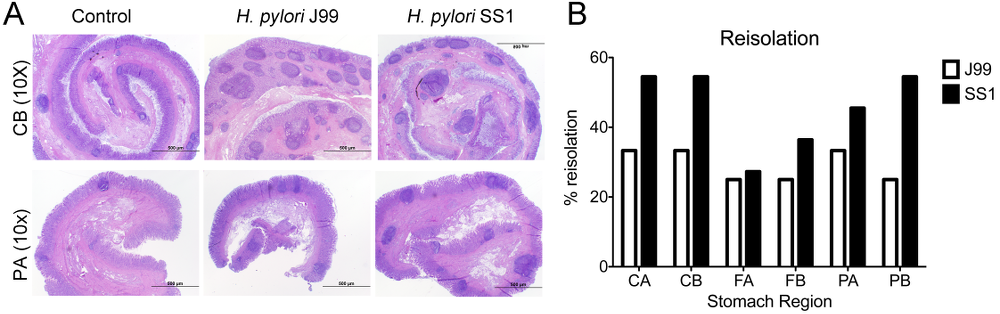 Figure 3. Lesion development upon H.pylori infection and the lack of bacterial clearance upon long-term infection with strain SS1.
Figure 3. Lesion development upon H.pylori infection and the lack of bacterial clearance upon long-term infection with strain SS1.
 Figure 1.
Figure 1.
 Figure 2.
Figure 2.
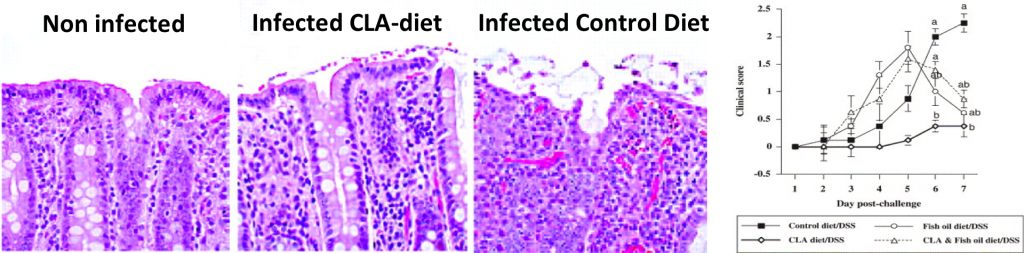 Figure 3.
Figure 3.
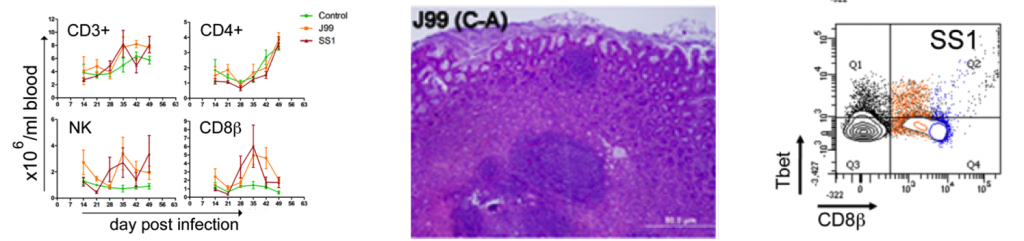 Figure 4.
Figure 4.
 Figure 5.
Figure 5.
 1. Preclinical models for Inflammatory Bowel Disease
1. Preclinical models for Inflammatory Bowel Disease
 2. Preclinical models for Infectious diseases: Respiratory pathogens
2. Preclinical models for Infectious diseases: Respiratory pathogens
 3. Preclinical models of Infectious diseases: Gastrointestinal pathogens
3. Preclinical models of Infectious diseases: Gastrointestinal pathogens
 4. Preclinical models to validate computational model approaches
4. Preclinical models to validate computational model approaches
 Computational Approaches: generating model predictions ready to validate
Computational Approaches: generating model predictions ready to validate